Ropidoxuridine
The phase I results have been reported by our SBIR subcontractor at the 30th EORTC-NCI- AACR Symposium in November 2018. Eighteen patients completed dose escalation to 1200 mg/day for 30 days, establishing the maximum tolerated dose (MTD) in combination with radiation therapy. Reported signals of efficacy include four patients with partial responses, nine with stable disease and one progressive disease in target lesions.
These results support advancing clinical development of Ropidoxuridine in combination with radiation and provide the foundation for design of phase Ib/II clinical trials in brain tumors and sarcomas. The brain tumor, glioblastoma is a disease site for which Shuttle Pharma received approval of an application to the FDA for orphan designations. Receiving orphan designation protects our marketing position of Ropidoxuridine for up to seven years after approval of drug commercialization. Our intellectual property also includes a provisional patent application for a new formulation.
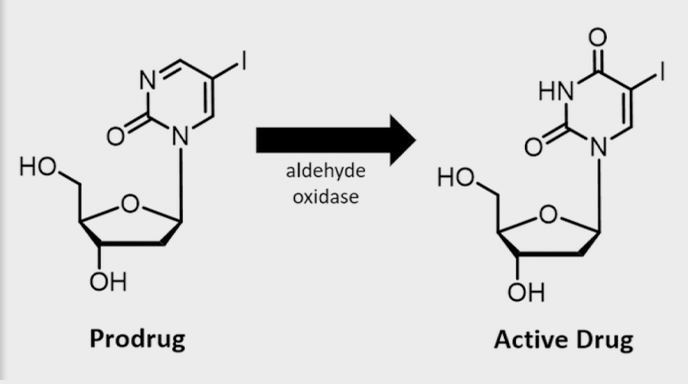
Extended Bio-availability Ropidoxuridine
Extended bio-availability Ropidoxuridine (IPdR/TPI) or Ropidoxuridine with Tipracil is a new combination formulation which increases the plasma levels of IUdR approximately 10-fold in pre-clinical testing. This new formulation is proposed for use in radiation sensitizing treatment of stage II and stage III rectal cancers with an endpoint of pathologic complete response rate (pCR) of greater than 40%, a surrogate of survival in patients with solid tumors. The new, extended bio- availability formulation of Ropidoxuridine/Tipracil will be advanced in parallel with other trials of Ropidoxuridine and radiation therapy.
Selective HDAC inhibitors
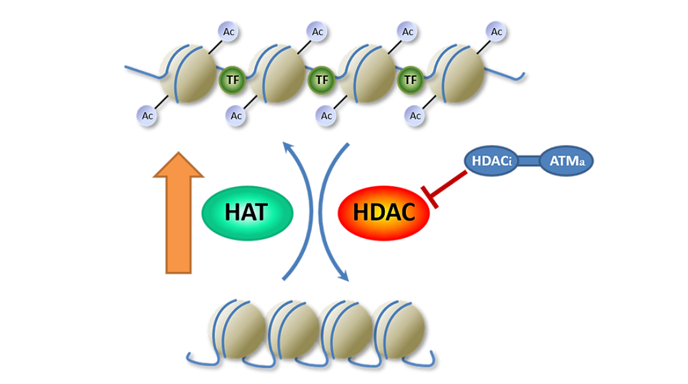
The current understanding of the roles of acetylation in the epigenetic regulation of chromatin structure, and gene expression rests on the balance of activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs). Increased acetylation of histones leads to changes in chromatin structure and accessibility for key cellular proteins to specific target sites.
HATs acetylate lysine groups at the amino terminal tails of nuclear histones to neutralize positive charges on the histones and yield a more open, transcriptionally active chromatin structure. In contrast, HDACs deacetylate, yield a condensed chromatin structure and suppress transcription. In this model, inhibitors of HDACs bias the balance toward a more acetylated state. Such as shift in the relative activities of these enzymes may change gene expression necessary DNA repair, replication, cell cycle checkpoint activation and tumor suppression. Acetylations of non-histone proteins also contribute to potential applications in cancer treatment. Our studies focus on selective HDAC inhibition to sensitize cancer to radiation therapy and to activate the anti-tumor immune responses.
SP-1-161
SP-1-161 is our leading pre-clinical product candidate and is a pan HDAC inhibitor that initiates the mutated in ataxia-telangiectasia (ATM) response pathway. ATM is activated by its phosphorylation at serine 1981 triggered by ionizing radiation induced DNA damage. Activated ATM phosphorylates critical factors involved in DNA repair, apoptosis, immunity regulation and cell cycle checkpoint. ATM can also be activated by HDAC inhibitors and by the radiation protective molecule, di-indolmethane. Using rational drug design, Shuttle scientists have discovered HDAC inhibitors and ATM activators capable of radiation sensitizing cancer cells and protecting normal cells of which SP-1-161 is our lead candidate. These candidate drugs may serve as direct chemotherapeutic agents or as radiation sensitizers for improving the outcomes of cancer treatment.
SP-2-225
SP-2-225 is a selective HDAC inhibitor that affects histone deacetylase HDAC6 and is a member of the class IIb HDAC family. HDAC6 is unique among HDAC enzymes in having two active catalytic domains and unique physiological function. In addition to the modification of histones, HDAC6 targets specific substrates including α-tubulin and HSP90, and is involved in protein trafficking and degradation, cell shape and migration. Selective HDAC6 inhibitors are an emerging class of pharmaceuticals due to the involvement of HDAC6 in pathways related to neurodegenerative diseases, cancer, and immunology. Specifically, its potential to affect regulation of the immune system and enhance the immune response in cancer is of great interest. With the introduction of CAR-T therapies and personalized medicine in cancer, regulation of the immune response to this therapy is of significant clinical and commercial interest.
